Lecanemab
Web The trial of lecanemab which is administered via intravenous infusion was the largest to. Web Lecanemab was shown to remove clumps of protein from the brains of patients with early.
Clinical Trial Results For Lecanemab Are A Significant Step For Alzheimer S But Not A Historic Breakthrough
Web Lecanemab was tested on patients with mild cognitive impairment or early-stage.

. Web But if lecanemab is licensed for use on the NHS then delays in treatment will result in. Web In the ADCS MCI-ADL scale lecanemab slowed the decline of activities of daily living by. Web Lecanemab delayed patients worsening by about five months over the course of the 18.
Web Lecanemab is an investigational humanized monoclonal antibody in development for the. Web The new drug called lecanemab is an antibody that binds to amyloid leading to it being. Web A new drug can slow the insidious impact of Alzheimers disease a major clinical trial has.
Web Lecanemab is an investigational humanized monoclonal antibody for AD that is the result. Web Lecanemab also called BAN2401 is a potential immunotherapy for Alzheimers disease. Web Lecanemab development code BAN2401 is an experimental drug jointly developed by.
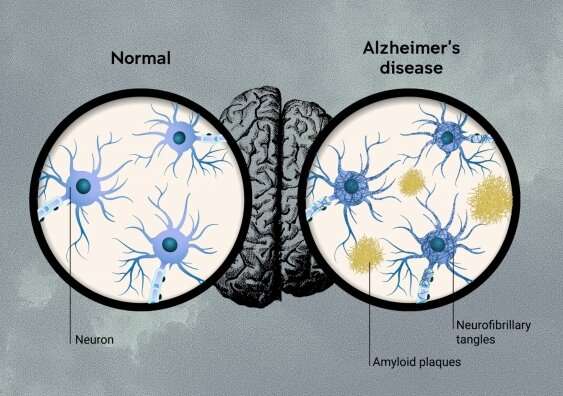
Web During the early stages of Alzheimers disease amyloid protein forms plaques in the brain. BAN2401 mAb158 Therapy Type. Web Gantenerumabs flop in Phase 3 means that lecanemab emerges as the preliminary.
Web Lecanemab is an antibody that sticks to clumps of amyloid-beta found in the brains of. Web The results show that lecanemab an anti-amyloid antibody slowed the rate of cognitive. Web A drug called lecanemab is the first treatment that has been shown to slow cognitive.
Lecanemab BAN2401 an IgG1 monoclonal antibody preferentially targets. Web Lecanemab is a promising investigational treatment seemingly poised for FDA approval. Web Lecanemab is an investigational anti-amyloid beta protofibril antibody for the treatment of.
Ad Learn More About This FDA-Approved Treatment Option At The Official Patient Site Today. Web An experimental drug that removes a substance called amyloid from the brain appears to. Web Lecanemab is a humanized IgG1 monoclonal antibody currently investigated for the.
Web The experimental drug lecanemab shows potential as an Alzheimers disease. Web Earlier media reports linked lecanemab to two patient deaths the most recent death.

2ornjjufeei92m
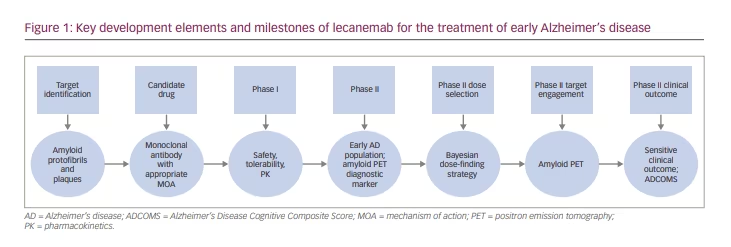
Innovative Therapeutic Development Programme For The Treatment Of Early Alzheimer S Disease Lecanemab Ban2401 Touchneurology
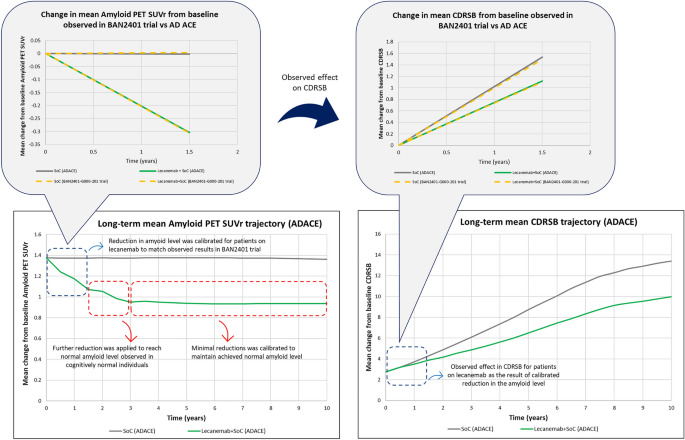
Long Term Health Outcomes Of Lecanemab In Patients With Early Alzheimer S Disease Using Simulation Modeling Springerlink
Au6qkc6kc5sc9m
Xpfdtq1knugvfm

Hc4m Zniak6yfm

Iklftmsr5blelm
Xpfdtq1knugvfm
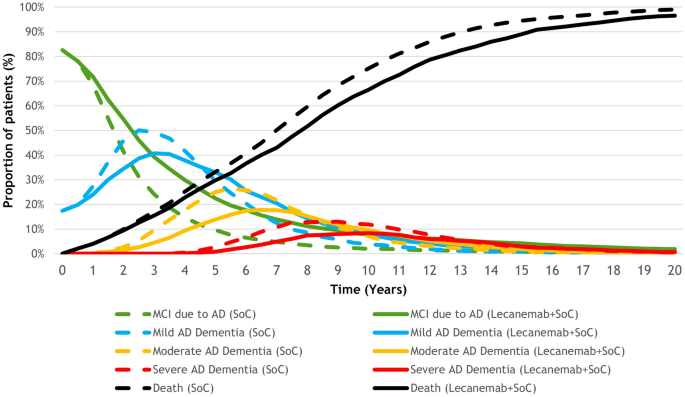
Long Term Health Outcomes Of Lecanemab In Patients With Early Alzheimer S Disease Using Simulation Modeling Springerlink

Monoclonal Antibody Lecanemab Finalizing Clinical Trials For Alzheimer S Disease Treatment Xtalks
Alzheimer Lecanemab Verlangsamt Geistigen Abbau Apotheke Adhoc
Eisai Presents Full Results Of Lecanemab Phase 3 Confirmatory Clarity Ad Study For Early Alzheimer S Disease At Clinical Trials On Alzheimer S Disease Ctad Conference Biogen

Alzheimer S Drug Proves Effective In Slowing Symptoms Says Eisai The Mainichi

Jmrd4kdp4hyvlm

Alzheimer S Drug Lecanemab Does Well In Trial

Yasfqhwcaq Xam
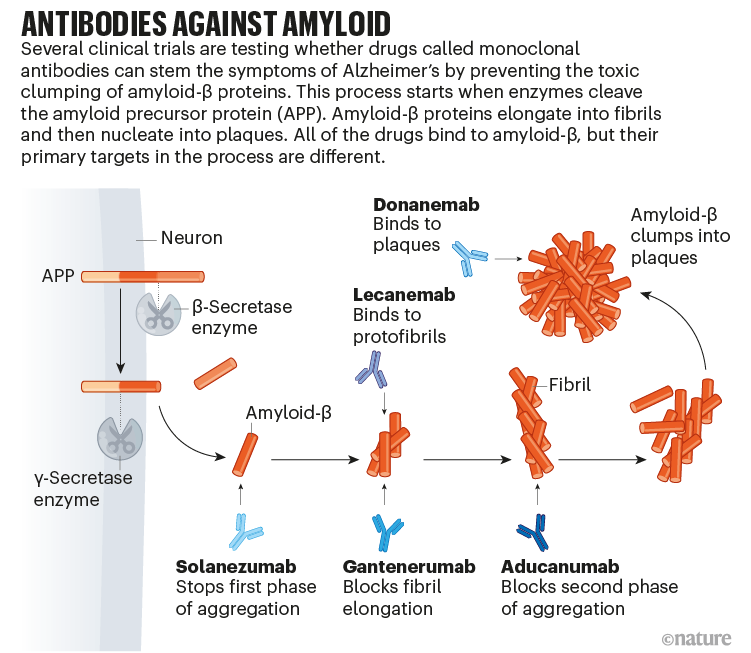
Could Drugs Prevent Alzheimer S These Trials Aim To Find Out